
The Science Behind Our Formulations: Why Mercy Means Trust
Introduce Mercy Life Science’s commitment to research-based, scientifically validated formulations. Discuss the role of GMP and ISO-certified manufacturing, formulation trials, and quality assurance.
How We Choose and Test Active Ingredients
At Mercy Life Science, the selection of active ingredients is driven by scientific evidence, clinical relevance, and global safety standards. Our team of pharmacologists and researchers conducts in-depth literature reviews and studies existing clinical trials to identify ingredients that are both effective and safe for human use. Once shortlisted, each active ingredient undergoes rigorous testing for identity, purity, potency, and stability in our controlled laboratories. We also evaluate supplier credentials and verify certifications to ensure the raw materials meet pharmacopeial standards such as IP, BP, or USP.

The Importance of Bioavailability and Safety
We believe that a formulation is only as good as its absorption and safety profile. That’s why we prioritize the bioavailability of each product—ensuring that nutrients and compounds are effectively absorbed and utilized by the body. Our formulations often include enhanced delivery systems such as nano-emulsions, micro-encapsulation, or synergistic co-factors to increase absorption. Every product is further screened for potential allergens, toxins, and heavy metals to meet strict safety parameters.
Collaboration with Expert Pathologists and Manufacturers
Our team collaborates closely with experienced pathologists, microbiologists, and lab technologists who lend their diagnostic expertise to product development. We also partner with India’s most reputable GMP and ISO-certified pharmaceutical manufacturers to bring these formulations to life. These collaborations ensure not only smooth manufacturing workflows but also the integration of clinical insight into product efficacy and safety.

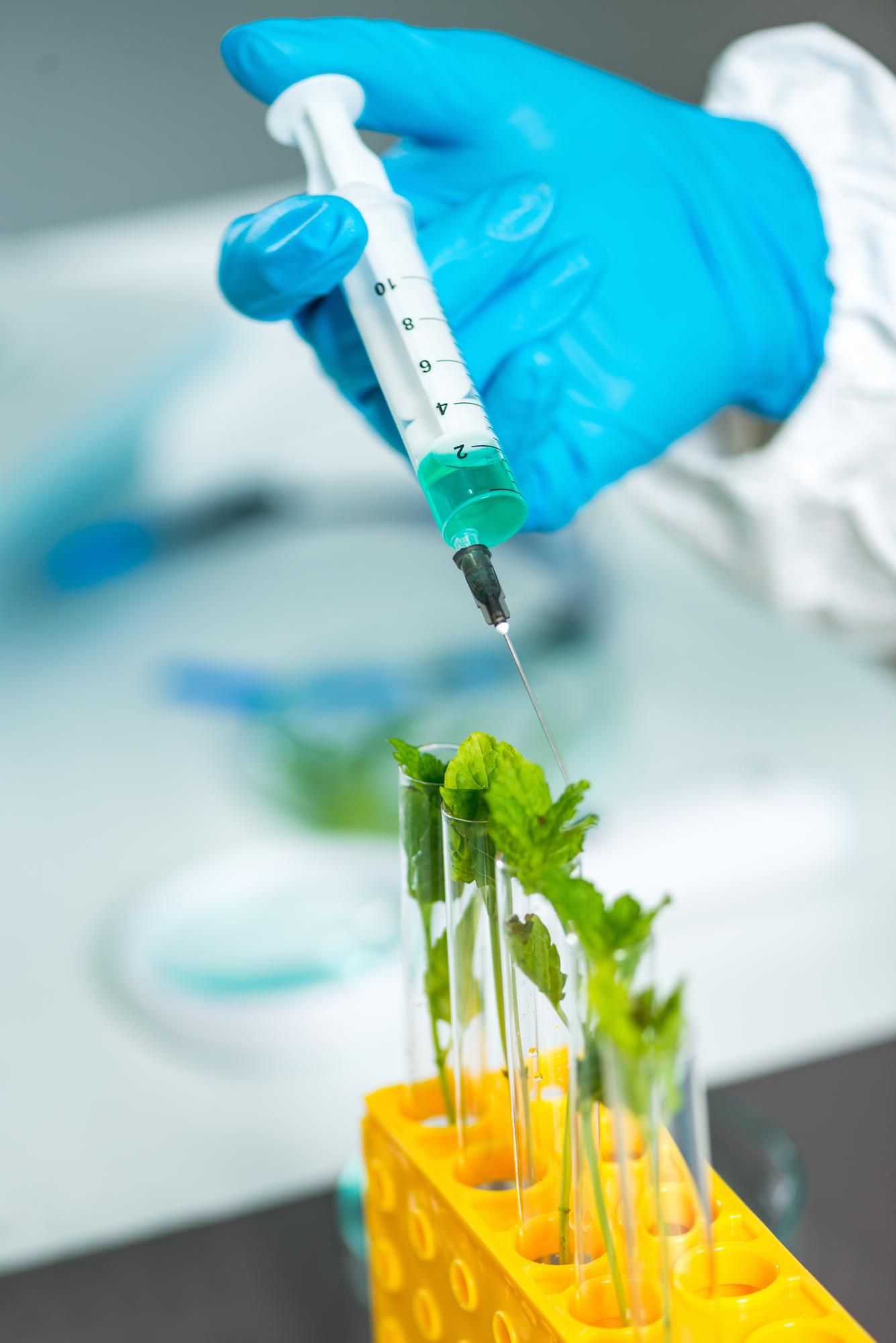

End-to-End Quality Control Protocols
From raw material intake to final packaging, our products pass through a multi-stage quality assurance process. These include:
Our in-house Quality Control (QC) and Quality Assurance (QA) teams work independently to certify that every batch meets or exceeds compliance with industry standards. No product leaves our facility without passing through these stringent checkpoints.
